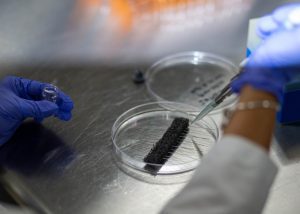
Medical Device Testing & Validation Services

To preserve the health and safety of patients and health-care professionals alike, it’s vital that medical device manufacturers and OEMs have access to thorough medical device testing services. Every piece of equipment used in the hospital environment must uphold the highest level of safety, quality, and performance standards.
To preserve the health and safety of patients and health-care professionals alike, it’s vital that medical device manufacturers and OEMs have access to thorough medical device testing services. Every piece of equipment used in the hospital environment must uphold the highest level of safety, quality, and performance standards. Device testing is a crucial part of the safety and health protocol for any lab. When you don’t know where to begin, consider reaching out to a knowledgeable medical device testing lab. HIGHPOWER is a leading validation and testing laboratory focusing on the complex and highly regulated world of reusable medical devices that require cleaning, packaging, and sterilization procedures.
With a broad global customer base and extensive regulatory experience (with the US FDA and other global regulatory bodies), our team provides full-service support throughout each phase of device design, validation, and regulatory approval. As a dedicated medical device testing lab, HIGHPOWER has both the resources and the flexibility to offer our clients tailored and personalized medical device validation services.
Browse our site to learn more about our comprehensive medical device testing and validation services. You can also contact us here to request an audit or ask any pressing questions.





 Biocompatibility
Biocompatibility