Medical device classifications organize different tools, tests, and other devices to help manufacturers design, produce, and sell safe, useful products. When it comes to reusable medical devices, different classifications influence how end users utilize, clean, and sterilize the products.
Understanding the different classes of reusable medical devices, known as the Spaulding Classification, helps manufacturers, validation labs, and health-care professionals make the most of different medical products and deliver safe, healthy solutions to patients. Learn more with this overview of reusable medical device classification and its influence on validation processes.
Defining Reusable Medical Devices
The purpose of reusable medical devices is to serve healthcare professionals and their patients over and over again. But in order for these devices to be effective, they need to be safe. To be safe, they need to be able to handle repetitive cleaning and sterilization processes between uses so that they’re ready for every new patient. Moreover, reusable medical device manufacturers need to know that their devices aren’t going to wear down and become inefficient, inaccurate, or ineffective as a result of repetitive sterilization processes.
Meeting these requirements takes time, care, and attention. Products need to undergo specific validation processes to ensure they’re safe to use. But before manufacturers can do that, they must classify the devices accurately to ensure they undergo the right testing processes. This is where reusable medical device classification comes in.
The Importance of Device Classification
Reusable medical device classification is part of the Food and Drug Administration’s categorization process. This classification system identifies the exposure risk of different reusable medical devices. After all, a tool that comes into contact with blood faces different contamination threats than a tool that only touches unbroken skin.
Categorizing medical devices according to their intended usage—and the contamination risk of that intended usage—helps manufacturers prioritize accurate and relevant validation services. This creates the most effective testing process possible and ensures that each product undergoes the right testing to prove its safety and efficacy before it hits the market.
Reusable medical device classification also affects the materials necessary in the device’s manufacturing process. For example, devices that have a higher risk of exposure and contamination typically consist of materials that are easier to sterilize and can stand up to intense and repetitive sterilization processes.
Proper device classification affects the entire manufacturing workflow and helps to ensure that every entity involved in production, validation, labeling, and distribution is on the same page about what each device needs and what purpose it fulfills.
Critical Devices
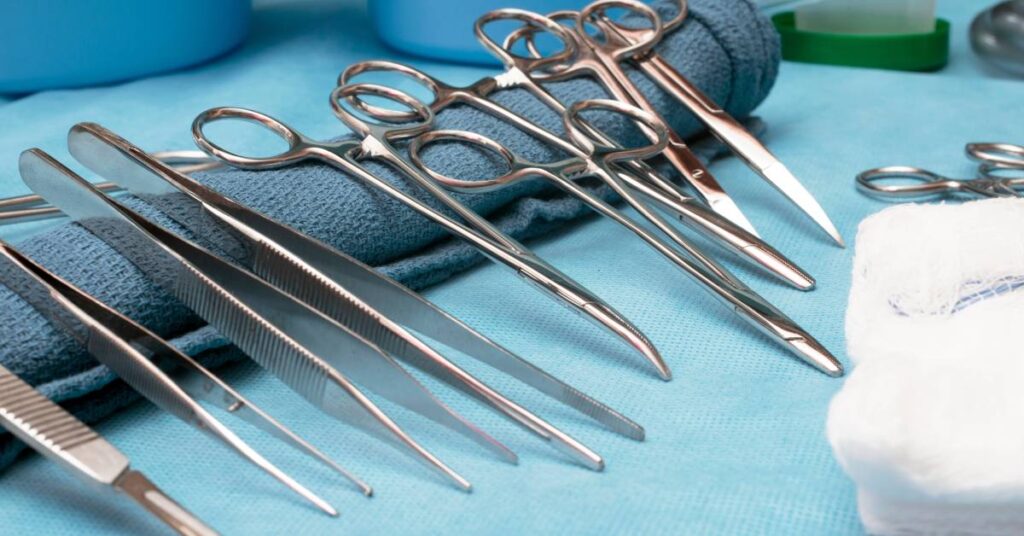
Critical devices make up the first class of reusable medical devices. Products in this category have the highest risk of exposure, infection, and contamination. These are the devices that typically come into direct contact with a patient’s bloodstream or internal tissues. These devices may have complex designs and interact with sensitive tissues, which means the risk of infection is extremely high if the devices aren’t sterile before usage.
Surgical instruments such as forceps, clamps, and scalpels are some of the most common examples of critical medical devices. Other critical devices include biopsy instruments, implants, and catheters.
Semi-Critical Devices
Semi-critical devices refer to reusable medical devices that have some contact with microbial materials and surfaces but do not interact with internal tissues or other sterile parts of the body. For example, a semi-critical device might come into contact with broken skin or intact mucus membranes. This type of contact holds some risk of contamination, but the risk isn’t as great as it is with critical medical devices.
Examples of semi-critical reusable medical devices include endoscopes, endotracheal tubes, laryngoscopes, cystoscopes, and anesthesia equipment.
Non-Critical Devices
Reusable medical devices with the lowest risk of contamination are known as non-critical devices. These types of tools come into contact with skin, but they do not penetrate the skin or body in any way.
Examples of these devices include stethoscopes, blood-pressure cuffs, ventilators, and skin electrodes. These devices operate on the outside of the body. However, because they are touching the patient, they still run a low risk of contamination through contact with blood, bodily fluids, and other sources of microorganisms.
Device Classification and Validation Processes
A reusable medical device’s classification helps to more precisely determine the specific processes necessary to remove contaminants so that it is safe to use again. This information plays a key role in cleaning validation tests, sterilization efficacy tests, and other crucial validation processes.
For all device classifications, manufacturers must make decisions based on worst-case microbial situations. This means that cleaning and sterilization processes must revolve around the assumption that the device has become as contaminated as possible for its classification during use. By working with the worst-case scenario during the design and validation processes, manufacturers ensure their products are safe to use in any situation that might occur once they’re in the hands of end-users.
Cleaning Validations
The cleaning validation process specifies a device’s cleaning guidelines and tests its efficacy to demonstrate how end users can and should remove contaminants between uses. Passing these tests makes sure that the cleaning guidelines are enough to properly disinfect the product. It also ensures that these cleaning procedures can occur between every use without prematurely wearing down the device and hindering its performance.
Cleaning validation also helps end users decide if and where they can use a certain medical device. For example, a doctor’s office can use the product’s cleaning instructions for use (IFU) to determine if they have the right equipment in their office to properly clean the device between uses.
Because cleaning processes precede sterilization processes, cleaning validation is crucial to the device’s sterilization. A proper cleaning process allows for a proper sterilization process, but an ineffective cleaning process can hinder the efficacy of sterilization and lead to possible cross-contamination and infection.
Sterilization Validations

Sterilization efficacy validation is the process of making sure that a device’s sterilization process eliminates harmful microorganisms, making it safe to use again. This form of testing involves putting the device through a specific sterilization process and then verifying that microorganisms have been eliminated to an appropriate sterility assurance level (SAL), which is typically 10-6. If the appropriate SAL is achieved, it proves the efficacy of the chosen sterilization method. If not, then manufacturers can refine their methods or their product design to achieve successful sterilization.
A device’s contamination risk affects how strict the sterility criteria are. For instance, reusable medical devices in the critical devices’ classification must meet higher standards of sterilization than non-critical devices, which may just require disinfection.
Validation Services From HIGHPOWER Labs
Cleaning and sterilization validation processes are important parts of regulatory compliance and quality control. These tests prove that the devices can perform safely and effectively time and time again in real-world medical settings. By completing these processes, medical device manufacturers produce products that are documented as being safe and reliable for health-care professionals and their patients before reaching the market.
Work with expert testing and validation teams when you partner with HIGHPOWER to validate your reusable medical devices’ reprocessing IFU. Learn more about our device cleaning validation testing services when you contact us today.