Sterilization is one of the most critical steps in ensuring patient safety in healthcare. The ability to reliably reprocess reusable medical devices is what allows hospitals and other healthcare providers to treat their patients safely and effectively. As a result, medical device reprocessing has become a vital process in maintaining a clean, safe environment for procedures ranging from routine checkups to major surgeries.
Despite its importance, improper sterilization of medical devices remains a significant problem in healthcare facilities worldwide, leading to harmful consequences for both patients and institutions. This blog will explore the negative effects of using improperly sterilized medical devices, outline the risks associated with this issue, and provide a clear understanding of the sterilization process that can safeguard health and prevent contamination.
Health Impacts of Improperly Sterilized Medical Devices
One of the most immediate and concerning impacts of using improperly sterilized medical devices is on patient health. When medical tools are not adequately cleaned and sterilized, they can become breeding grounds for pathogens. This results in direct patient exposure to harmful microorganisms, increasing the risk of post-procedure infections.
For example, surgical instruments that retain organic matter like blood or tissue can introduce these contaminants into patients’ bodies, causing surgical site infections (SSIs). These infections are not only painful and debilitating but can also lead to life-threatening complications, prolonged hospital stays, and higher mortality rates.
Beyond the physical pain and suffering, patients can also experience long-term health issues and impaired recovery due to infections caused by contaminated medical devices. This highlights the critical role that proper sterilization plays in protecting patient well-being.
Risks of Infection and Disease Transmission
One of the most alarming consequences of improper sterilization is the potential for disease transmission. Without comprehensive and reliable sterilization procedures, reusable medical devices can act as vectors for transmitting infections between patients. Bloodborne pathogens, including viruses like hepatitis B, hepatitis C, and HIV, are among the most dangerous threats posed by contaminated surgical instruments or needles.
Facility-acquired infections, often referred to as healthcare-associated infections (HAIs), are another significant concern. Many of these infections arise from the improper sterilization of reusable devices. Multidrug-resistant organisms are especially troubling, as they pose an added challenge to treatment and recovery.
The implications of such risks extend beyond the immediate healthcare setting. Patients with infections spread by improperly sterilized devices may unknowingly pass these to family members or communities once discharged. This can create broader public health challenges and increase the strain on healthcare systems to manage disease outbreaks. Proper sterilization protocols are essential not only to individual patient safety but also to preventing the systemic spread of infections.
Risks to Healthcare Workers

The dangers go beyond just patients, as unsafe sterilization practices can jeopardize healthcare workers as well. These risks arise during direct handling of contaminated devices, especially during and after surgeries and treatments. Accidental injuries like needle sticks or cuts can introduce harmful pathogens into a worker’s bloodstream, posing long-term health threats. Improper sterilization does not discriminate, creating a chain of exposure that affects everyone within the medical environment.
Legal and Financial Risks for Healthcare Facilities
The negative effects of improperly sterilized medical devices extend beyond health consequences; they can result in substantial legal and financial repercussions for healthcare providers. Hospitals and clinics have a legal obligation to provide a safe environment for patients, including ensuring the proper sterilization of medical devices. When they fail in this responsibility, they open themselves up to lawsuits and regulatory penalties.
Malpractice lawsuits related to infections or health complications can stem from contaminated devices and inadequate reprocessing procedures. Hospitals found to be negligent in maintaining sterilization standards may face significant settlements, reputational damage, and loss of public trust. Healthcare regulatory bodies often impose fines and sanctions for violations of sterilization protocols, further compounding the financial burden on institutions.
More broadly, improper sterilization can lead to indirect costs like canceled surgeries, increased demand for infection control resources, and extended hospital stays for infected patients. These risks underscore the importance of investing in proper staff training, equipment maintenance, and adherence to reprocessing guidelines to protect institutions from preventable liabilities.
Preventing Harm with Proper Sterilization
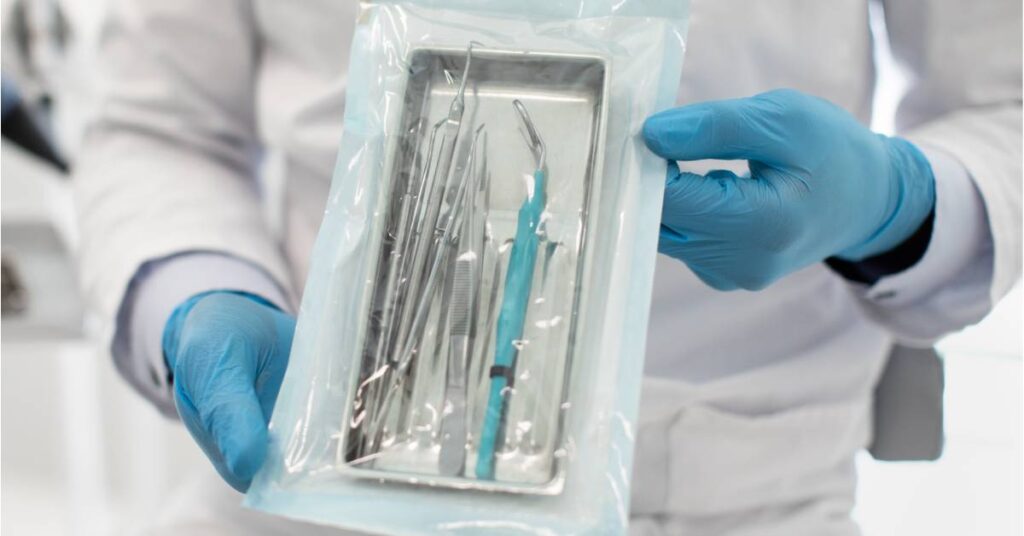
To ensure that reusable medical devices are safe for patient use, healthcare facilities must follow a meticulous and regulated reprocessing protocol. This multi-step process is designed to eliminate contaminants, prevent the spread of pathogens, and maintain device functionality. Every step of this process plays a vital role in delivering effective infection control and ensuring patient safety.
The first step in reprocessing is point-of-use treatment, which occurs immediately after the device is used. Healthcare professionals must remove gross contaminants like blood or bodily fluids at the procedure site to prevent debris from hardening later. Next is the cleaning phase, where devices undergo mechanical or manual cleaning with detergents to dislodge any remaining organic matter. Cleaning is always performed before sterilization to prevent the spread of microorganisms and ensure safe handling of the device during the rest of the reprocessing procedure.
Following cleaning, devices undergo inspection and preparation to check for damage or wear that may compromise their performance or sterility. Any necessary repairs or replacements are performed at this stage. Proper device assembly is then followed by packaging, where each instrument is securely wrapped to maintain sterility after the process is complete.
The central aspect of reprocessing is sterilization, during which devices are treated with methods such as steam, ethylene oxide gas, or vaporized hydrogen peroxide sterilization. Each technique is chosen based on the device’s material and susceptibility to heat or moisture. Proper monitoring and validation ensure that the reprocessing procedure consistently meets sterilization requirements and adequately prepares the device for eventual reuse.
Finally, reprocessed devices are stored in sterile storage under controlled conditions to protect them from contamination until they are ready for use. Every phase of this carefully regulated process is essential for ensuring that medical devices are both safe and functional when needed.
The Role of Education and Training in Sterilization Practices
Education and training play a vital role in maintaining proper sterilization practices. Staff must be well-versed in the latest guidelines and techniques to avoid costly mistakes.
Conducting regular training sessions and certifications increases both awareness and competency among employees responsible for sterilization. Leadership must prioritize fostering a culture of accountability, ensuring that all team members understand the life-and-death stakes of their work. Periodically reviewing sterilization efforts can provide an added layer of oversight and reinforce good practices.
Sterilization Validation With Highpower
Proper sterilization processes serve as the backbone of safe healthcare. The dangers stemming from improperly sterilized medical devices are entirely preventable through vigilance, technology, and education.
At Highpower Labs, we’re committed to validating adequate sterilization processes for reusable medical devices. Learn more about how we bring assurance and compliance to reusable medical device development with our medical device packaging validation and other sterilization validation services.