Reliable sterilization testing is the key to ensuring reusable medical devices are safe for use in a healthcare setting. Without validated sterilization methods, it is impossible for healthcare facilities to successfully reprocess medical devices and prevent cross-contamination between patients.
Because sterilization testing plays such a vital role in healthcare, validating these processes requires precise and accurate testing systems. That is where biological indicators come in. Read on to learn more about what biological indicators are and the role they play in sterilization testing.
What Are Biological Indicators?
Biological indicators are a way of testing and proving the efficacy of a sterilization process for a reusable medical device. This test system contains viable microorganisms that are resistant to the sterilization process you are validating. By observing the level of microorganisms present before and after the sterilization process, validators can confidently determine whether the process will successfully make a medical device safe for reuse. Biological indicators are used in sterilization validation processes involving steam, hydrogen peroxide gas, ethylene oxide, and other methods. Biological indicators used in healthcare are nonpathogenic organisms (not harmful). Most bacteria are nonpathogenic.
There are three vital characteristics of a biological indicator to pay attention to: purity, population, and D-value. Purity is the phenotypic identification of the specific bacterium species that is the reference microorganism for that test. The most common bacterial spores for biological indicator tests are the genus Bacillus, Geobacillus, and Clostridium.
Population refers to the target population at the end of the sterilization process. This population determines the microbial survival probability of the chosen sterilization method and indicates how likely it is for sterilization to successfully make the device safe for reuse. For most biological indicators, the target population is greater than 1 times 106.
The D-value is the parameter of sterilization to reduce the microorganism population by 1 log, or 90 percent of the population. This value also indicates the level of resistance the microorganism has to the sterilization parameters.
The Importance of Resistance
Biological indicators specifically use microorganisms that are hard to kill with the specific method of sterilization they are validating. This, along with practices such as applying abnormally high levels of microorganisms and placing biological indicators in hard-to-reach locations during the sterilization process, contributes to worst-case condition testing. This “overkill” method verifies that the sterilization process for a reusable medical device is able to effectively kill a safe number of organisms even in less-than-ideal conditions. Sterilization processes that pass this form of validation ensure greater safety and success in a real-world clinical setting.
Bacterial spores are the primary form of microorganisms for biological indicators because of their resistance. Validation processes will also test microorganisms that are specifically resistant to the chosen method of sterilization. For example, Geobacillus stearothermophilus spores are highly resistant toward heat and some types of chemicals, making them valuable in biological indicators testing steam and vaporized hydrogen peroxide sterilization methods. These are the two most popular sterilization methods currently used in healthcare settings.
The FDA Guidance document on 510K submissions for Biological Indicators list the typical spores for sterilization methods as follows:
Sterilization Process
Spore (Indicator Organism)
STEAM
Geobacillus Stearothermophilus
(formerly Bacillus stearothermophilus)
DRY HEAT
Bacillus Atrophaeus
(formerly Bacillus subtilis var. niger)
ETHYLENE OXIDE
Bacillus Atrophaeusn
(formerly Bacillus subtilis var. niger)
HYDROGEN PEROXIDE
Geobacillus Stearothermophilus
(formerly Bacillus stearothermophilus)
Spore resistance can stem from a variety of factors. Intrinsic resistance revolves around genetic factors that allow the bacterial endospores to survive in environments such as heat, high pressure, and radiation. Extrinsic resistance, on the other hand, refers to conditions beyond the endospores themselves. This includes factors such as the carrier or device material you are testing, the location of the microorganisms on the device, the preparation of the biological indicators, or the storage environment.
How Biological Indicator Testing Works
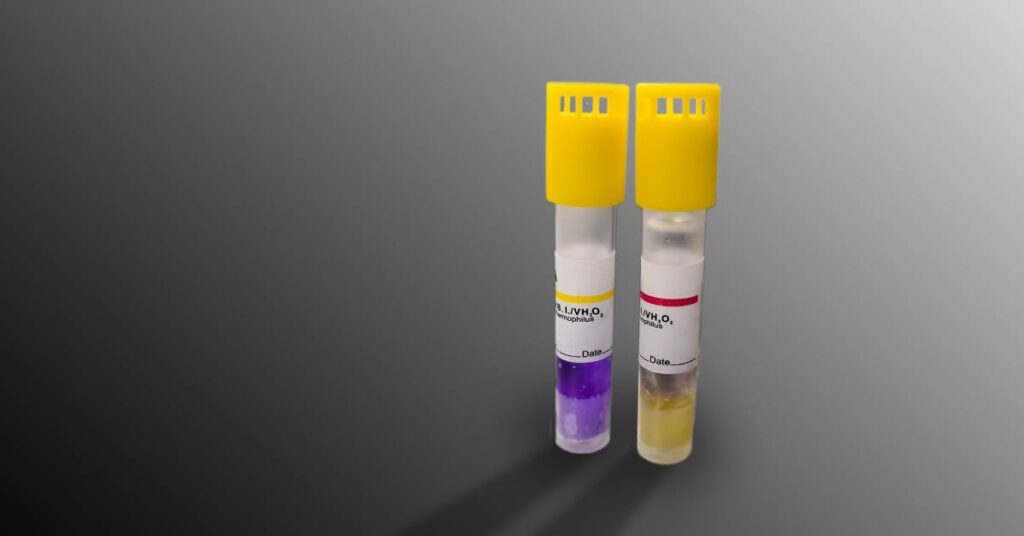
There are basically, three variations of biological indicators used in validation work.
- The first form uses spores added to a carrier such as filter paper or glass (spore strip), placed within primary packaging (glassine, Tyvek etc.) designed to maintain the integrity and viability of the inoculated carrier.
- The second form is a suspension of spores that is inoculated into or onto the product being sterilized (known as direct inoculation) or placed on a suitable carrier such as a wire, thread, suture, stainless steel disc etc.
- The third type consists of a package that includes both the microorganisms (spores) to be exposed, and also a growth medium (self-contained & sealed glass ampule) to recover the organisms after the sterilization process.
Professionals using the 3rd type, begin the testing process by applying the spores to the carrier or actual device. To simulate worst-case scenario testing, they use higher levels of biological indicators than would occur in average clinical settings and place the carrier or devices in the autoclave or other sterilization process in a less effective location.
After the sterilization process has ended, professionals incubate the biological indicator under specific growth conditions to monitor and determine spore growth. If spore growth occurs, it means the sterilization process did not reach all of the target microorganisms, indicating a failed process and a failed test. However, if zero spore growth occurs during the incubation period, it indicates that the sterilization process killed a sufficient number of microorganisms, thus passing the test and validating the sterilization method.
Biological Indicators Offer Precision
Biological indicators play an important role in sterilization testing because they create a way to specifically and precisely measure the success of a sterilization process. Every sterilization method has variables—including operator errors, variations in how someone prepares the device for sterilization, the condition of the equipment, and so on.
However, biological indicators provide an objective analysis on the microbial level. This offers reliable assurance of the sterilization method’s success, making it a key part in safely validating sterilization processes for a reusable medical device.
Using Biological Indicators in Steam Sterilization

Steam sterilization is one of the most popular and dependable methods of sterilization for reusable medical devices. Conservative estimates are that 80-90% of all loads processed in healthcare facilities are done so in the steam process. When testing steam sterilization processes and equipment with biological indicators, it is important to place the biological indicator in the least effective location of the sterilization load, as placed in the sterilization chamber. This poses a greater challenge for the sterilization process and provides greater assurance if the test succeeds. For steam sterilizers, this least effective location is usually on the bottom shelf of the chamber, near the drain.
Using Biological Indicators in Vaporized Hydrogen Peroxide Sterilization
Biological indicators also work with chemical sterilization processes such as vaporized hydrogen peroxide sterilization. This sterilization method often involves the ability to run multiple cycles within the sterilizer. As such, the biological indicator must undergo validation testing for each cycle.
Producing and Validating Biological Indicators
Biological indicators play a key role in validating sterilization processes or device manufacturers instructions for use (IFU) and ensuring the safety and efficacy of reusable medical devices in a healthcare setting. At Highpower, we offer verification and validation services for population, resistance and purity to ensure biological indicators are meeting their performance requirements.
Our decades of experience give us the knowledge and the resources necessary to help successfully produce, test, and validate biological indicators. We base our methodology on industry standards from the United States Pharmacopeia/National Formulary, ANSI/AAMI, and ISO 11138, ensuring a dependable and compliant validation process for your business.
Find Validation Services With Highpower Labs
Highpower has over 35 years of experience in testing and validating sterilization for medical devices. Explore our service options today and see how we can help you bring your device successfully to market.