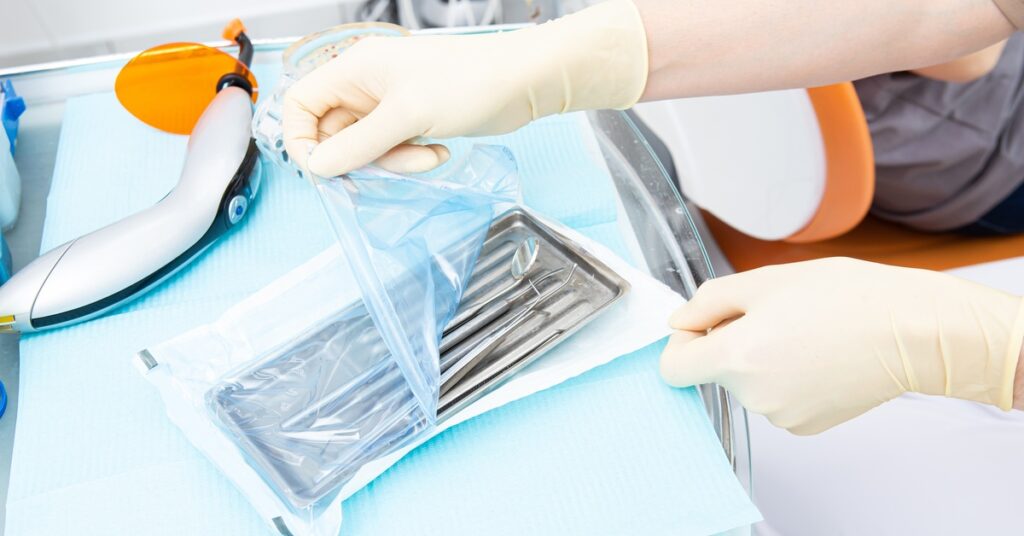
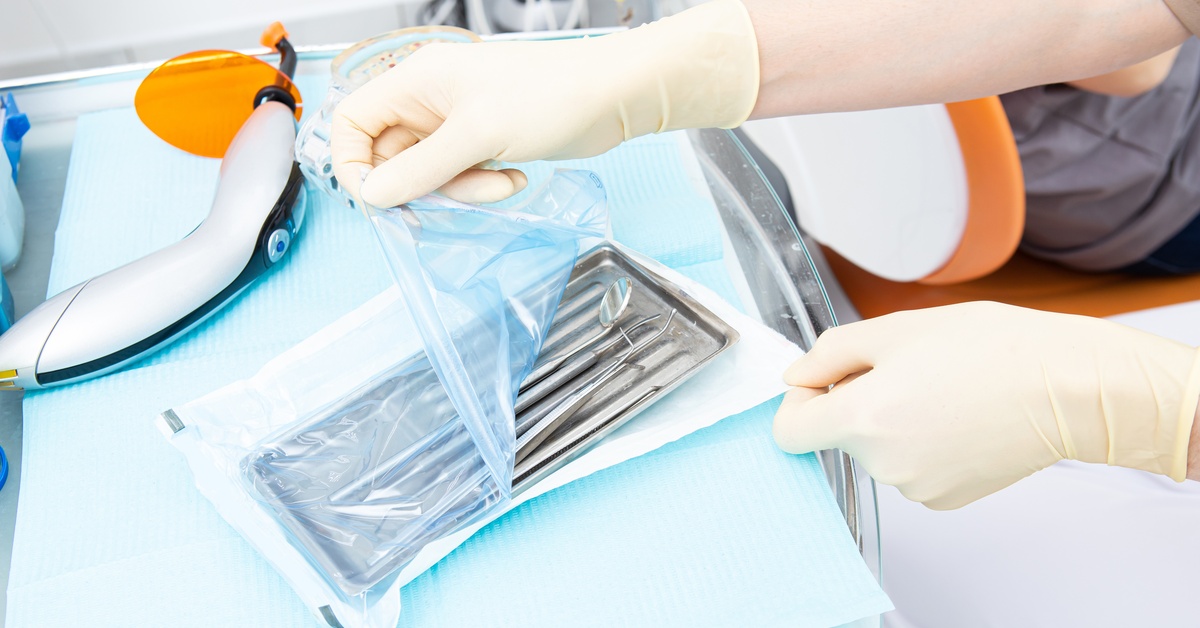
When it comes to reusable medical devices, proper packaging is a non-negotiable component of ensuring effective reprocessing and delivering sterile solutions to end users. Effective terminal medical device packaging ensures that medical devices remain untainted from the moment they leave the healthcare facilities reprocessing department until the moment they’re used in critical medical procedures.
Validation is the process that confirms a device’s packaging system meets stringent regulatory standards for safety, sterility, and performance. Yet, packaging failures during validation are all too common, causing costly delays, wasted resources, and potential risks to patients.
Understanding why such failures occur can help medical device manufacturers avoid these setbacks and maintain compliance with regulatory standards. Below, we’ll explore the key reasons why medical device packaging often doesn’t pass validation and how you can address these challenges proactively to achieve market approval for your device.
Failure to Maintain the Sterile Barrier
The sterile barrier system is the backbone of medical device packaging, and its failure results in immediate rejection during validation. The sterile barrier protects the reusable medical device and prevents contamination during reprocessing and storage. This ensures that the device is safe for use when healthcare professionals need it.
From selecting compatible materials to ensuring proper sealing, every aspect of your packaging design must prioritize the sterile barrier. The design needs to uphold the barrier under various conditions, including transportation, storage, and sterilization.
Common points of failure include weak seals, punctures, or materials that degrade over time. Even small breaches in the sterile barrier can lead to contamination risks.
Poor Material Choice
One of the most common reasons medical device packaging fails validation comes down to the materials used. Choosing the right packaging materials is absolutely critical, as they must stand up to a variety of conditions while maintaining the integrity of the medical device within.
For example, packaging materials need to resist heat, moisture, and other environmental stresses encountered during sterilization, transportation, or storage. Often, manufacturers underestimate the environmental challenges their packaging will face, leading to cracks, tears, or degraded performance during testing.
Sterile barrier systems rely on materials that can create secure seals while still allowing for easy opening without compromising sterility. Beyond that, these materials must also demonstrate biocompatibility, ensuring they do not negatively interact with the medical devices they’re designed to protect. Shelf life is another essential factor. Materials must be able to preserve their sterility and physical properties over a long duration, depending on the intended use of the product.
Failing to take these aspects into account or opting for cheaper, substandard materials can quickly lead to failed validation attempts. In fact, a reputable validation lab with experience in packaging systems can even predict the outcome of the testing based on a review of the product, its country of origin and the packaging systems specifications.
Incorrect Packaging Size
Size is another critical consideration when it comes to medical device packaging. Packaging that is either too large or too small can spell disaster during validation. Incorrectly sized packaging leads to improper sterilization, flawed sealing, compromised sterile barrier systems, and potential damage to the device within.
Terminal sterilization packaging is commonly divided into three types: sterile wraps, sterile pouches, and rigid containers. Each of these has its own sizing requirements that must align perfectly with the dimensions of the medical device in question. Packaging that’s too small may stretch or compress the contents, which can puncture the sterile barrier or prevent proper sterilization. On the other hand, oversized packaging can affect sealing and allow contaminants to penetrate.
Validation often catches these mistakes, and resolving sizing issues can mean going back to the drawing board. Manufacturers must conduct thorough testing early on to ensure their packaging can adequately fit and protect the devices without compromising sterility or usability. It can also be advantageous to perform initial feasibility testing on the packaging system prior to initiating a full validation.
Labeling Mistakes
Labeling is a critical area where mistakes can lead to failed packaging validation. Labels on medical device packaging must meet rigorous regulatory requirements to ensure they provide clear and accurate information to healthcare providers.
Regulations such as those outlined by the FDA mandate that medical device labels include key details, such as the manufacturer’s name and address, product descriptions, expiration dates, storage conditions, and instructions for use. Mistakes such as improperly printed labels, poor readability, unclear instructions, or even missing details can raise red flags during validation.
Labels also need to withstand the same environmental conditions as the packaging itself. Smudged or deteriorated labels can make critical information unreadable, and that’s enough to fail validation. Manufacturers must maintain airtight processes to ensure their labels remain compliant and legible throughout the product’s lifecycle.
Cutting Corners in Design and Manufacturing
The pressure of meeting tight deadlines or reducing production costs can tempt some manufacturers to cut corners during the design and manufacturing of packaging. While this might save time in the short term, it often leads to long-term issues that arise during validation.
Skipping key steps such as rigorous testing or quality control checks often results in packaging that’s unable to meet regulatory requirements. Some manufacturers may also rush through material selection, neglect essential compatibility tests, or downplay the importance of prototyping. Even if a shortcut saves time initially, it quickly becomes evident during validation, causing expensive delays and reputational damage.
A robust design and manufacturing process is non-negotiable in the medical device industry. Manufacturers must commit to a step-by-step approach that emphasizes precision, quality, and compliance, rather than speed alone.
Failure to Meet Regulatory Requirements
Medical device packaging must adhere to strict regulations, including FDA and ISO guidelines. These requirements ensure packaging not only protects the device but also complies with safety and performance criteria that prevent risks to patients and healthcare professionals.
Examples of non-compliant packaging might include improper labeling, use of non-certified materials, or failure to ensure sterility over the designated shelf life. In addition to failed validation, non-compliance can result in fines, product recalls, and serious risks to patient safety.
To avoid these pitfalls, manufacturers must stay up to date with evolving regulatory requirements and perform ongoing audits of their raw material purchasing and storage, quality inspections and manufacturing processes. Compliance must be viewed as a foundation rather than an obstacle, as aligning with these standards ultimately ensures the safety and reliability of your product.
A Commitment to Better Packaging
Medical device packaging failures during validation represent a significant challenge, but they are far from insurmountable. Understanding and addressing the common pitfalls can help manufacturers build robust, reliable packaging that stands up to validation and real-world use.
If you’re looking for thorough, expert medical package validation testing, turn to the experts at Highpower Validation. Learn more about our shelf-life tests, package integrity tests, and other critical validation tests when you talk to our team today.