In the medical device industry, packaging ensures the efficacy of the products and the safety of the patients who use them. Medical device packaging must protect the product from damage and contamination during transportation and storage. It must also maintain sterility and provide necessary information to healthcare professionals and patients so they can use the device correctly.
Because of its importance, medical device packaging goes through rigorous testing to pass inspection and gain approval for use. When a packaging design fails inspection, it leads to product delays or delays in scheduled surgeries, increased costs, and potential harm to patients. Learn more about the most common reasons why medical device packaging fails inspection and how manufacturers and hospital workers can avoid these mistakes.
1. Using the Wrong Packaging Materials
One of the main reasons for packaging failures is the use of incorrect materials. Medical devices have specific requirements for packaging based on factors such as sterility, shelf life, and environmental conditions. For example, devices that are sensitive to moisture require packaging with moisture-resistant properties.
Choosing materials that do not provide the necessary protection can compromise the sterility and functionality of the device, leading to inspection failures. Additionally, using materials that are incompatible with sterilization processes, such as plastics that cannot withstand high temperatures, can result in failed inspections.
2. Using the Wrong Packaging Container
The shape and size of packaging are just as crucial as the materials they consist of. Containers that are too large can lead to excess device movement during transit, potentially damaging the product. It can also cause folds, wrinkles, or creases, which can compromise the sterile barrier system of the package.
Conversely, containers that are too small may apply pressure on the device and compromise its integrity. Manufacturers must carefully evaluate the shape and size of packaging in relation to the device to ensure it meets all functional and regulatory requirements.
3. Inadequate Sterile Barrier System
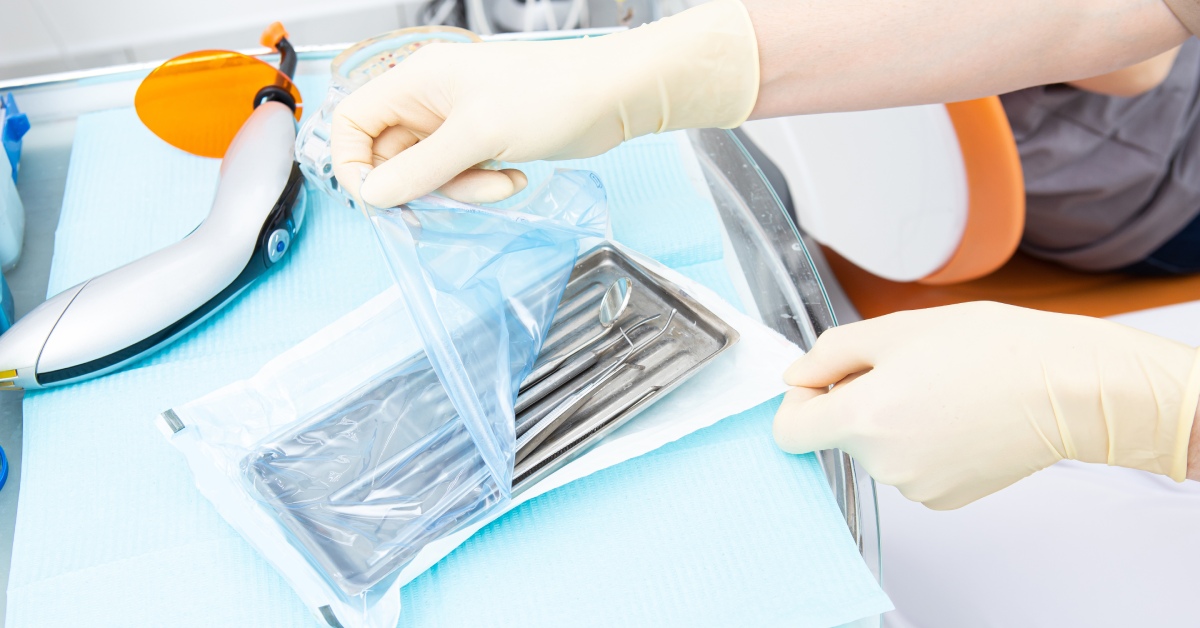
One of the most critical aspects of successful medical device packaging is a sterile barrier system that protects the product during transport and storage. Maintaining sterility is a non-negotiable aspect of medical device packaging; even the slightest breach in sterility can lead to contamination and potentially endanger patient safety.
When medical devices move through the supply chain, the exterior of the packaging inevitably becomes contaminated by environmental factors such as moisture, dust, and microorganisms. A sterile barrier system creates a seal that keeps those contaminants from affecting the interior of the packaging and the product within. It also prevents the non-sterile packaging exterior from contaminating the medical device when the end user opens the product.
To maintain sterility throughout the supply chain, the sterile barrier system must withstand typical handling and transportation stress. This requires durable packaging materials to prevent tears or punctures that break the sterile barrier. Packaging designs also need strong seals that prevent leaks and other unwanted breaches. Performing quality checks during the sealing process can prevent issues such as inconsistent sealing pressure, contaminated heat seal devices, and inconsistent sealing equipment controls that create weak, ineffective seals.
4. Incorrect Labeling or Missing Information
Product and packaging labeling are the basis of communication across the supply chain. Labels provide information such as the device’s name, manufacturer details, expiration date, usage instructions, and safety information. Incorrect or missing information can have serious consequences, including misuse of the device or non-compliance with regulations.
Incorrect information is the most common cause of labeling mistakes. A single typo or misprint can completely alter vital details such as expiration dates or dosage instructions. Because of this, it is crucial to pay close attention to every step of the labeling process. Comprehensive quality management systems can help workers identify and correct minor errors to prevent major problems.
Additionally, regulatory bodies have labeling requirements that logistics teams must adhere to. Failing to meet these standards can result in inspection failures. Manufacturers must ensure that all labels are clear, accurate, and compliant with industry regulations. This involves regular checks and updates to labeling processes, as well as training for staff involved in the labeling procedures.
5. Poor Packaging Design
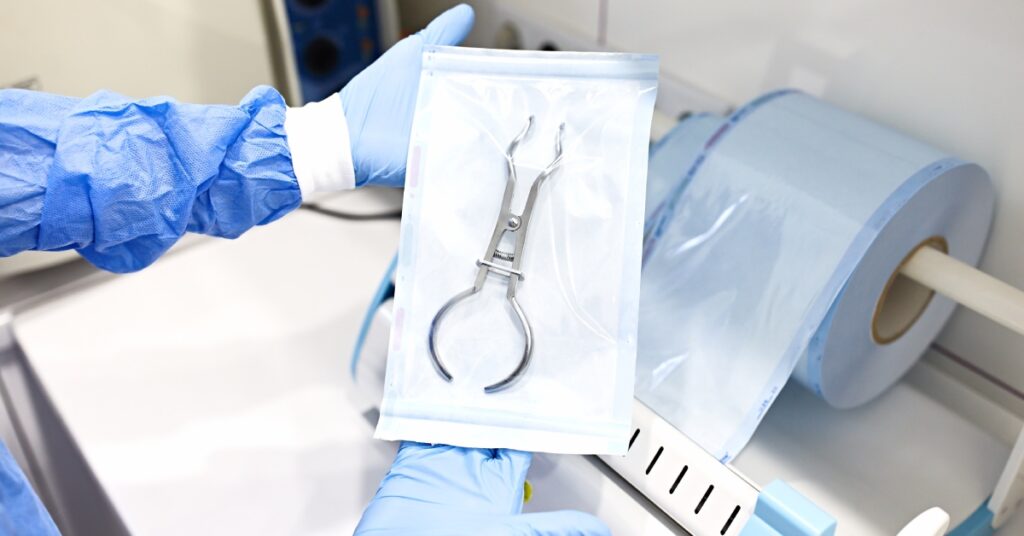
Another common reason for medical device packaging inspection failures is poor packaging design. Medical device packaging designs must protect the contents during handling and transportation. However, if the designers don’t consider the needs of the device and the activities in the supply chain, the packaging could fail to provide sufficient protection.
For instance, if the packaging is too rigid, it may not absorb shocks during transit, leading to potential damage to the device. Conversely, if the packaging is too flexible, it might not offer enough stability, causing the device to shift and sustain damage. Additionally, packaging that is too complex or difficult to open can lead to mishandling, further increasing the risk of damage.
To prevent these issues, manufacturers must conduct thorough testing and validation during the design phase to ensure that the packaging can withstand the various stresses it will encounter throughout its lifecycle.
6. Non-Compliance With Industry Regulations
The medical device industry is highly regulated to keep patients safe. Packaging designs must meet stringent requirements to ensure safety and efficacy. These regulations cover everything from material selection and design to labeling and sterilization processes. Ignoring them or failing to stay on top of updates can lead to non-compliance and failed inspections.
Manufacturers must be proactive in keeping abreast of changes to regulations for medical device production. They should also implement quality control systems to ensure ongoing compliance. Regular audits, comprehensive quality management systems, and ongoing training programs can help in maintaining high standards and preventing non-compliance issues.
The Importance of Medical Device Packaging Validation
Before a product hits the market, manufacturers must conduct rigorous testing to validate the packaging design. Validation ensures the packaging can maintain device sterility throughout its shelf life under various conditions.
Comprehensive packaging validation involves testing for seal integrity, material compatibility, and environmental robustness. Through simulations and integrity tests, manufacturers can confirm their packaging design provides adequate protection for their products, setting themselves up for success during the approval process.
Highpower Labs offers comprehensive and compliant medical device packaging validation. Learn more about our services and how we can validate your design when you talk with our expert team today.
