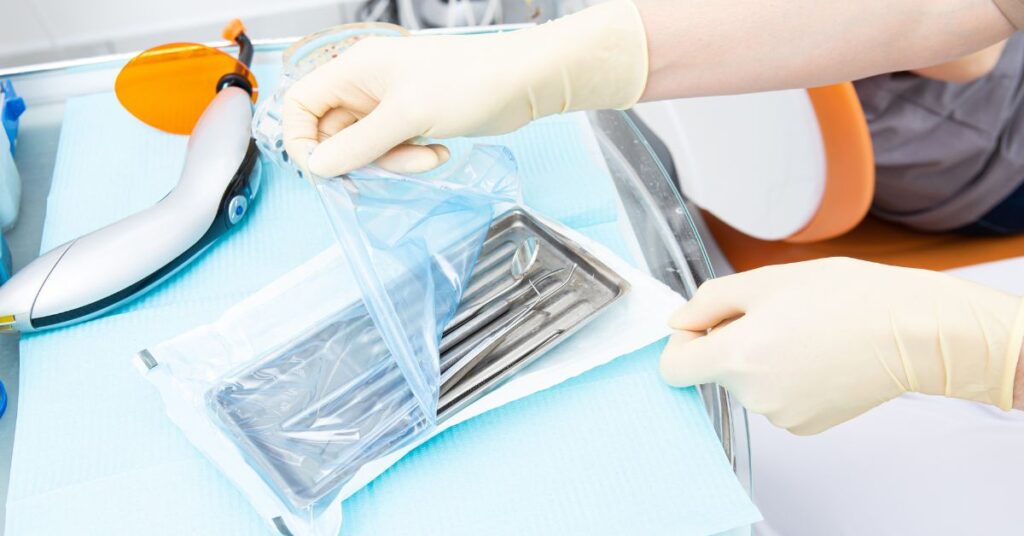
Packaging plays a key role in keeping reusable medical devices sterile and safe as they are stored after use or as they travel throughout supply chains after sterile processing. Without secure, sterile packaging, contaminants from storage within healthcare facilities and transport vehicles could taint the efficacy of the medical devices. This contamination could lead to infection, putting healthcare facilities, device manufacturers, and, most importantly, their patients at serious risk. Medical device packaging validation involves performing package integrity tests and other real-world condition testing to ensure these devices remain sterile until point of use by healthcare professionals.
Validating packaging systems requires a system design that will protect and preserve the integrity of the contents within. This requires carefully selecting the format and materials for your medical device packaging. Learn more about what goes into selecting and designing the right packaging system with this guide to the different types of medical device packaging formats and materials.
Formats and Materials for Terminal Medical Device Packaging
There are a few types of packaging systems for reusable medical devices in healthcare facilities, and the ones commonly validated by HIGHPOWER are sterilization pouches, sterilization wraps, and rigid container systems. Selecting the right format involves considerations such as the size of the device, intended storage procedures for the device, the suspected shelf life of the packaged device, the type of labeling necessary, and whether the packaging itself is reusable or single-use. The packaging format can also influence features such as seals, tamperproof closures, and more.
Choosing a format that is compatible with your medical device and its use is key to creating packaging that maintains sterility and provides adequate protection throughout storage and transportation.
Sterilization Pouches
Sterilization pouches are flexible packaging systems that create a seal around the device to preserve the sterile barrier and maintain the integrity of the packaging. It is crucial for sterilization pouches to use sealing methods that do not compromise the sterility of the packaging design. This means avoiding methods such as staples or pins and opting for methods of closure such as heat sealing when utilizing heat seal pouches or following proper sealing procedures when using self-seal pouches. Some medical devices—particularly heavy or sharp instruments—call for double sealable pouches for extra strength and integrity.
Additionally, when designing pouch systems for sharp reusable medical devices, it is crucial to package the devices in a way that will not puncture the pouch and break the sterile barrier. Tip protectors and other validated packaging methods can prevent this and maintain the integrity of the packaging system.
Sterilization pouches come in multiple sizes and weights, such as 60 g/m, to meet the demand of the healthcare market they are targeting, be it the dental or medical market. A heavier paper stock typically results in a more durable pouch. The two most common sterilization pouches used in healthcare settings are paper/plastic and Tyvek/plastic sterilization pouches.
Tyvek
Tyvek is a flexible, paper-like material that offers incredible durability for medical grade sterilization pouches. Its breathability prevents condensation buildup and helps devices maintain a longer shelf-life without contamination or device degradation. It is also extremely tear-resistant, making it a strong choice that can hold up against jostling and other movements during storage and transport. Tyvek has the ability to maintain a strong microbial barrier, but it also has a clean peel, allowing for aseptic removal of devices when end users’ open the packaging.
Another significant advantage of Tyvek is its compatibility with many common sterilization methods, including low-temperature steam, ethylene oxide, hydrogen peroxide, and other low-temperature oxidative methods. This makes it a versatile packaging material that can serve a wide range of medical devices.
Paper
Paper is a less expensive alternative to Tyvek and offers good durability for use in both medical and dental sterilization pouches. It is breathable and helps maintain the pouches’ shelf-life without contamination or device degradation. However, it does tear more easily than Tyvek, especially when wet, but once dry, it holds up well against jostling and other movements during storage and transport. Kraft paper has the ability to maintain a good microbial barrier and has a clean peel, allowing for aseptic removal of devices when end users open the packaging.
Paper is compatible with common sterilization methods, including steam and ethylene oxide. This makes it a versatile, less expensive packaging material that can serve a wide range of medical devices.
Sterilization Wraps
There are two main wrapping techniques for sterilization wrap described in the AAMI standards: simultaneous and sequential. Both techniques can be closed with either an envelope or a square folding style. Wraps can be a single layer or double-bonded and are made of either a woven or non-woven material. The AAMI standards tell us to wrap with two layers of sterilization wrap under most circumstances, although a single wrap may be appropriate for “immediate use steam sterilization”. Ultimately, the goal of each wrapping method is to maintain sterility during storage, transport, and as the end user removes the device from the packaging materials.
Sterilization wraps use specific tape to adequately secure the wrap. This tape must be compatible with the packaging system’s sterilization method, be it steam, ethylene oxide, or hydrogen peroxide. It must also be able to withstand all the parameters of the sterilization process, such as the chemical composition of the sterilant, as well as the pressure, heat, and moisture associated with the sterilizing agent and its intended transportation and storage practices. The two most common sterilization wraps used in healthcare settings are historically known as SMS and CSR wrap.
SMS Wrap
SMS wrap is a non-woven, multi-layered fabric that is common in various sterilization wrap packaging systems. It consists of a layer of spun-bond (S) polypropylene, then a layer of melt-blown (M) polypropylene, and then a final layer of spun-bond polypropylene. This creates a breathable, water-resistant fabric that is flexible enough to wrap around medical device trays and other products, but durable enough to create and maintain a sterile barrier. SMS comes in multiple sizes and weights to meet the demands of the healthcare market.
SMS wrap offers good filtration, high tensile strength, and a strong bacterial barrier. It works for both single- and double-wrap packaging systems. It is also compatible with multiple common sterility methods, including steam, ethylene oxide, and hydrogen peroxide, and is most commonly used in hospitals and surgery centers.
CSR Wrap
Central supply room (CSR) wrap is a soft, non-woven fabric typically made with a crepe paper that offers a strong barrier against airborne and waterborne bacteria. This flexible packaging material features high tensile strength when wet or dry. It also repels water and provides excellent durability against wear and tear. CSR wrap comes in multiple sizes and weights to meet the demand of the alternative care healthcare market. CSR fabric is compatible with both steam and ethylene oxide sterilization processes and is most commonly used in dental and physical office settings.
Rigid Container Systems
Rigid container systems are a heavy-duty form of packaging that offer solid, strong barrier protection for medical devices. Many rigid container systems include tamperproof closures and locking mechanisms, making it easier to preserve sterility and identify when the sterile barrier is broken. These systems can also include dividers, mats, reusable or disposable filters, corner protectors, padding, and other materials or features to protect medical instruments and optimize safe, sterile storage procedures. They are compatible with multiple common sterilization methods, including steam, ethylene oxide, and hydrogen peroxide. They are most commonly used in hospitals and surgery centers, and the two most common rigid container materials are aluminum and plastic composites.
Aluminum
Aluminum is a popular packaging material across a wide range of industries. When it comes to reusable medical devices, aluminum offers both strength and flexibility and allows for quicker drying times in steam sterilization cycles. The sturdiness of aluminum means that it is less likely to bend, crack, dent, or take other damage during transport and storage. This helps preserve the sterile barrier, protect the contents within the packaging, and prolong the shelf life of the packaging system.
Plastic
Plastic and its composites are popular packaging materials across a wide range of industries. When it comes to reusable medical devices, plastic offers both strength and flexibility and can be lighter than comparable metal rigid containers. However, plastic can have extended drying times in steam sterilization cycles. It works well in immediate use steam sterilization (IUSS) rigid containers.
Validating Packaging Systems
HIGHPOWER Labs has over 30 years of experience in developing, testing, and validating different packaging formats and materials for reusable medical devices. We have the testing equipment, practices, and knowledge necessary to ensure your designs protect and preserve the intended medical devices in real-world environments. Learn more about our medical device package testing when you contact the experts at HIGHPOWER Labs today.