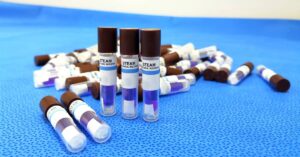
Biological indicators help prove the efficacy of a sterilization process. Explore the role of biological indicators in sterilization testing with this guide.…

A Look at the Global Medical Device Regulatory Authorities
It’s important to know what regulations you face as you bring a device to market. Learn about two global medical device regulatory authorities with this guide.
…

How Packaging Can Determine a Medical Device’s Shelf Life
Sterile packaging means a longer and safer storage period. Learn more about how packaging determines a medical device’s shelf life with this guide.…

Comparing Types of Validations for Medical Device Packaging
Packaging validation determines a packaging system’s ability to maintain sterility. Compare types of terminal medical device packaging validations with this guide.…
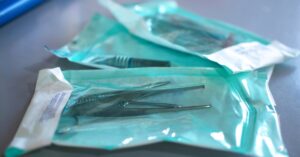
A Look at the Reprocessing of Reusable Medical Devices
Reusable medical devices must undergo reprocessing to prevent contamination between uses. Learn more about what that process looks like with this overview.…
What Is the Difference Between Half- and Full-Cycle Testing?
There are various sterilization tests available to ensure effective device reprocessing. Learn about the difference between half- and full-cycle testing.…