It’s important to know what regulations you face as you bring a device to market. Learn about two global medical device regulatory authorities with this guide.
…


It’s important to know what regulations you face as you bring a device to market. Learn about two global medical device regulatory authorities with this guide.
…
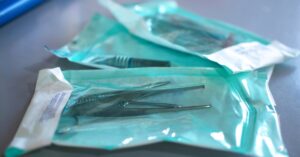
Reusable medical devices must undergo reprocessing to prevent contamination between uses. Learn more about what that process looks like with this overview.…

Medical device classification ties into market regulations and approval. Learn about the classification process of medical devices in the US with this guide.…

Cleaning validation tests are integral to the development and approval of reusable medical devices. They play a key role in establishing proper reprocessing standards so…

Sterilization validation plays a key role in developing safe medical devices. Learn how sterilization efficacy is tested in medical devices with this guide.…

Proper medical packaging protects end users and their patients. Explore the different types of medical device packaging formats and materials.…